|
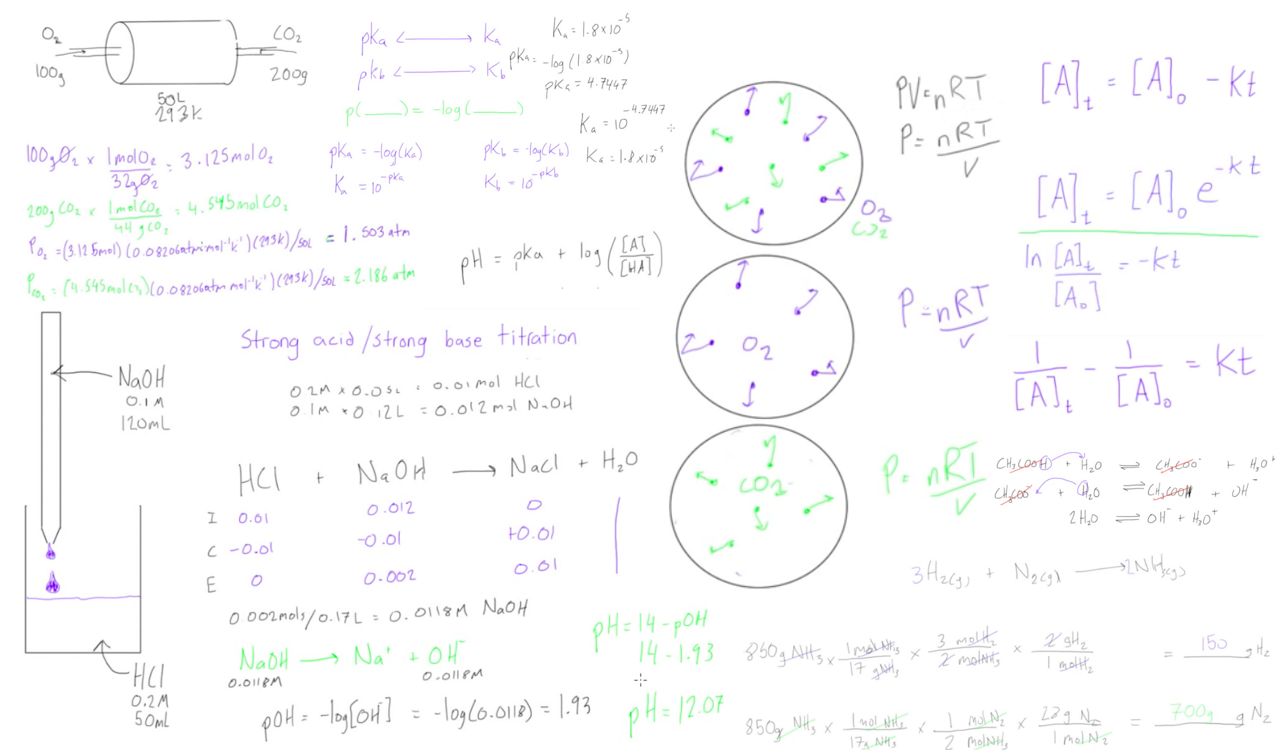
This free online chemistry course teaches everything you need to know to succeed in chemistry. The tutorials cover everything from converting between Ka and pKa to acid/base titrations to the very basics of stoichiometry. The course consists of 33 tutorials which cover the material of a typical first year general chemistry course at the university level.
In order to gain a comprehensive understanding of the subject, you should start at the top and work your way down the list. That way you will start learning the most basic concepts first and build off of those as you progress through the course. |
|
© Copyright www.engineer4free.com 2012 - 2024 All Rights Reserved
About | Course List | Patreon | Newsletter | Blog | ToS | Contact Engineer4Free is committed to sustainability. You should be too. |
|